Primary Treatment – Removing Bulk Pollutants: Lecture 2
Overall Goal: To understand the fundamental physical and chemical processes involved in primary treatment of textile dyeing and pretreatment wastewater, and their effectiveness in removing major pollutants.
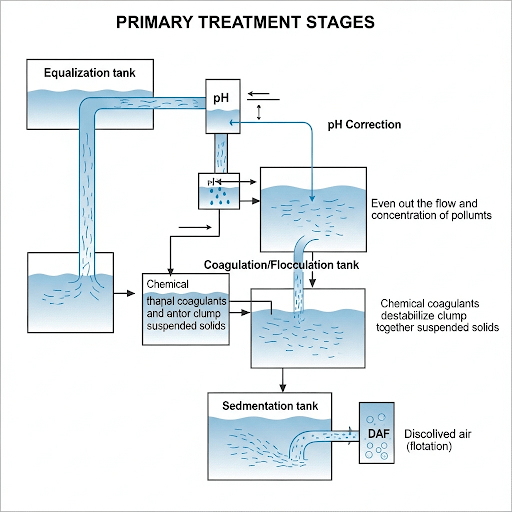
2.1. Equalization & pH Correction: Setting the Stage
- The Problem of Variability: Textile wastewater isn’t uniform. It changes dramatically in flow, concentration of pollutants, and especially pH, depending on the specific processes running in the factory. This variability is a nightmare for downstream treatment units.
- Equalization Basins:
- Purpose: These are large tanks designed to buffer these fluctuations. They mix incoming wastewater to create a more consistent flow and pollutant load for subsequent treatment stages. Think of it as a “balancing act” for the wastewater.
- Design Considerations: We’ll look at how the size (volume) of an equalization tank is determined based on factory production patterns and desired retention time. We’ll also discuss the importance of adequate mixing to prevent settling and ensure homogeneity.
- pH Adjustment:
- Why it’s Crucial: Most biological and chemical treatment processes operate optimally within a specific pH range. Textile wastewater can swing from highly acidic (e.g., from bleaching) to highly alkaline (e.g., from mercerizing or dyeing).
- How it’s Done: We’ll cover the use of chemicals like sulfuric acid (H2SO4) to lower pH and sodium hydroxide (NaOH) or calcium hydroxide (Ca(OH)2) to raise pH, and the automated systems used for precise control.
2.2. Coagulation & Flocculation: Making the Unsettleable Settle
- The Challenge: Many pollutants in textile wastewater, especially dyes and fine suspended particles, are very stable and won’t settle out on their own. They might have a negative charge, causing them to repel each other.
- Coagulation:
- Principle: This is the rapid mixing step where a coagulant chemical is added. Coagulants (e.g., Alum (Al2(SO4)3), Ferric Chloride (FeCl3), or Polyaluminum Chloride (PAC)) work to destabilize these stable particles, often by neutralizing their electrical charges. This allows the tiny particles to start clumping together.
- Mechanism: We’ll delve into the concepts of charge neutralization, adsorption, and bridging.
- Flocculation:
- Principle: After coagulation, the water moves into a flocculation tank where gentle, slow mixing occurs. This promotes the collision and aggregation of the destabilized particles into larger, heavier, and more easily settleable clumps called flocs.
- Optimizing the Process: The Jar Test: We’ll explore this essential laboratory procedure used to determine the optimal type and dosage of coagulant and flocculant, as well as the ideal pH for maximum pollutant removal.
- Pollutant Removal: This combined process is highly effective in significantly reducing color, Total Suspended Solids (TSS), and a considerable portion of the Chemical Oxygen Demand (COD) associated with suspended and colloidal matter.
2.3. Sedimentation / Clarification: Separating the Solids from the Water
- The Goal: Once the flocs are formed, the next step is to physically separate them from the water.
- Sedimentation Tanks (Clarifiers):
- How they Work: These are large basins where the water flow velocity is significantly reduced, allowing gravity to pull the heavy flocs down to the bottom.
- Types: We’ll look at common designs like conventional circular clarifiers with rotating scrapers and lamella clarifiers, which use inclined plates to provide a larger settling area in a smaller footprint.
- Sludge Handling: The settled material at the bottom is known as primary sludge. We’ll discuss its collection and the next steps for its management.
2.4. (Optional) Dissolved Air Flotation (DAF): An Alternative for Lighter Pollutants
- When to Use DAF: While sedimentation removes heavy particles, some pollutants, like oils, greases, and certain dyes, might be lighter than water or have a lower density, making them difficult to settle. DAF is particularly effective for these.
- Principle: Very fine air bubbles are introduced into the wastewater under pressure. These bubbles attach to the suspended particles, increasing their buoyancy and causing them to float to the surface, forming a scum layer that can be skimmed off.
- Advantages: Can achieve higher clarity than sedimentation for certain types of wastewater and can be more compact.
By the end of this lecture, you’ll have a clear understanding of how the initial physical and chemical processes in primary treatment lay the groundwork for more advanced wastewater purification, preparing the effluent for biological treatment.
