Textile Pretreatment: Lecture #6 – Mercerization
Welcome back to our series on Textile Pretreatment! We’ve systematically moved through cleaning processes like greige fabric inspection, singeing, desizing, and scouring, culminating in bleaching for whiteness. Now, we arrive at a specialized and transformative pretreatment step for cotton: Mercerization.
1. Introduction to Mercerization
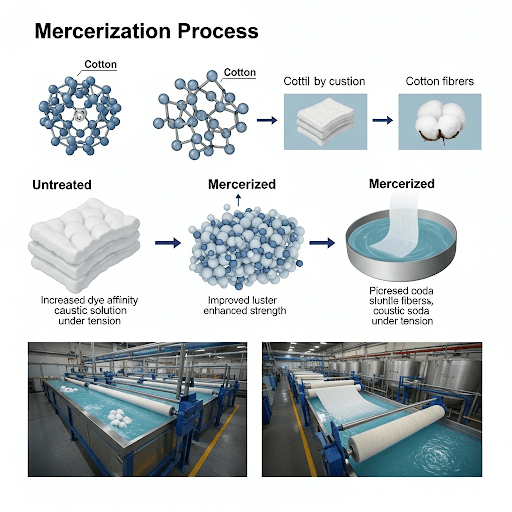
Mercerization is a chemical treatment for cellulosic fibers (primarily cotton) with a concentrated caustic soda (sodium hydroxide, NaOH) solution under tension, or sometimes without tension. It’s named after John Mercer, who discovered the process in 1844.
Why is Mercerization Essential (for Cotton)?
While not always a mandatory step, mercerization is performed to significantly enhance several desirable properties of cotton, particularly for high-quality fabrics:
- Increased Tensile Strength: Mercerized cotton is 15-20% stronger than unmercerized cotton. This makes the fabric more durable and resistant to tearing.
- Enhanced Luster (Sheen): Unmercerized cotton fibers are kidney-bean shaped and have a rough surface, scattering light. Mercerization causes the fibers to swell, become more circular in cross-section, and develop a smoother surface, which reflects light more uniformly, imparting a permanent, silk-like luster or sheen.
- Improved Dye Uptake/Dye Affinity: The structural changes within the fiber make it more reactive and porous, allowing dyes to penetrate and fix more efficiently. This results in deeper, brighter, and richer shades with less dye consumption and improved dye fastness.
- Increased Dimensional Stability: Mercerization under tension helps to stabilize the fabric dimensions, reducing shrinkage during subsequent washing.
- Improved Smoothness and Hand Feel: The smoother fiber surface contributes to a softer and more pleasant hand feel.
- Increased Absorbency: While already achieved through scouring, mercerization further enhances the fiber’s absorbency due to increased porosity.
Essentially, mercerization transforms ordinary cotton into a superior material with enhanced aesthetic and performance characteristics.
2. The Chemistry and Mechanism of Mercerization
Mercerization involves a physical and chemical transformation of the cellulose within the cotton fiber.
- Cellulose Transformation: Cotton cellulose exists primarily in a crystalline structure known as Cellulose I (native cellulose). During mercerization, the concentrated caustic soda solution penetrates the amorphous and semi-crystalline regions of the fiber, causing the cellulose molecules to rearrange into a more stable and accessible crystalline structure called Cellulose II. This transformation is largely irreversible.
- Fiber Swelling: The NaOH solution causes significant swelling of the cotton fibers. The initially kidney-bean shaped cross-section becomes more round or oval, and the fiber lumen (the central canal) either shrinks or disappears. This swelling opens up the fiber structure, increasing its porosity and internal surface area.
- Increased Reactivity: The rearrangement to Cellulose II and the increased accessibility due to swelling expose more hydroxyl groups (−OH) in the cellulose, making the fiber more reactive to dyes and chemical finishes.
- The Role of Tension:
- Under Tension (Most Common): When mercerization is carried out with the fabric held under controlled tension (longitudinal and sometimes transverse), the swelling fibers are prevented from shrinking lengthwise. This tension aligns the cellulose molecules, increasing fiber strength, enhancing luster (by creating a smoother, more uniform surface), and preventing fabric shrinkage. This is known as “Lustre Mercerization.”
- Without Tension (Less Common, for special effects): If cotton yarn or fabric is mercerized without tension, it undergoes significant shrinkage, leading to a bulky yarn with improved bulk and dye uptake, but without the increased luster. This is sometimes called “Stretch Mercerization” or “Slack Mercerization.”
3. The Mercerization Process
Mercerization is typically carried out on continuous ranges due to the high volume of fabric and the need for controlled tension.
Key Stages in a Mercerizing Machine:
- Impregnation: The fabric is passed through a cold, concentrated solution of caustic soda (typically 20-30% NaOH). The temperature is kept low (15−25∘C) to facilitate swelling and penetration of the NaOH. A wetting agent may be added to aid rapid and uniform penetration.
- Dwelling/Reaction Zone: The fabric remains immersed or saturated with the caustic solution for a specific dwell time (e.g., 30-60 seconds) to allow the swelling and chemical transformation to occur fully.
- Tensioning: This is the most crucial stage for luster and strength. The fabric is held under significant and consistent tension by a series of rollers or clips (in a chain mercerizer) as it moves through the treatment zone and into the washing sections. The tension is maintained while the caustic soda is still active on the fiber.
- Washing: While still under tension, the fabric is thoroughly washed with hot and then cold water. This step is critical to remove the caustic soda from the fiber. Washing while under tension helps to “lock in” the desired structural changes and prevents shrinkage.
- Neutralization: The fabric is then passed through a dilute acid bath (e.g., sulfuric acid, acetic acid) to neutralize any residual alkali. This ensures the fiber’s pH is brought back to neutral, preventing degradation and preparing it for subsequent dyeing.
- Final Washing: A final rinse to remove the acid and any remaining impurities.
4. Factors Influencing Mercerization Efficiency
- Caustic Soda Concentration: Optimal range is 20-30% NaOH. Too low, and swelling is insufficient; too high, and it can be difficult to wash out.
- Temperature: Low temperatures (below 25∘C) are preferred as they promote maximum swelling and penetration without excessive fiber degradation.
- Tension: Critical for luster, strength, and dimensional stability. Must be uniform across the fabric width.
- Time: Sufficient dwell time is needed for complete penetration and reaction.
- Wetting Agents: Aid in rapid and uniform penetration of the caustic solution.
- Fiber Purity: Fabric must be thoroughly scoured and bleached before mercerization, as impurities can hinder uniform penetration and reaction.
5. Environmental and Safety Considerations
- Caustic Soda Handling: Highly corrosive chemical, requires stringent safety protocols (PPE, ventilation, emergency showers).
- Effluent: High pH wastewater containing residual NaOH. Requires neutralization before discharge.
- Energy and Water Consumption: Mercerization is an energy and water-intensive process.
- Caustic Recovery: Large mercerization plants often employ caustic recovery systems to concentrate and reuse the NaOH solution, reducing both chemical consumption and effluent load.
Mercerization is a sophisticated and highly beneficial pretreatment step for cotton textiles, enhancing their quality and performance for a wide range of applications, especially in home textiles, apparel, and high-quality shirting.
