Textile Dyeing and Dyes: Lecture #16 – A Deeper Dive into Dye Chemistry – Part 2: Direct Dyes
Building upon our understanding of Azo dye chemistry, let’s now focus on Direct Dyes. These anionic dyes are characterized by their ability to dye cellulosic fibers (like cotton, linen, and viscose) directly in an aqueous solution, typically with the addition of electrolytes (salts).
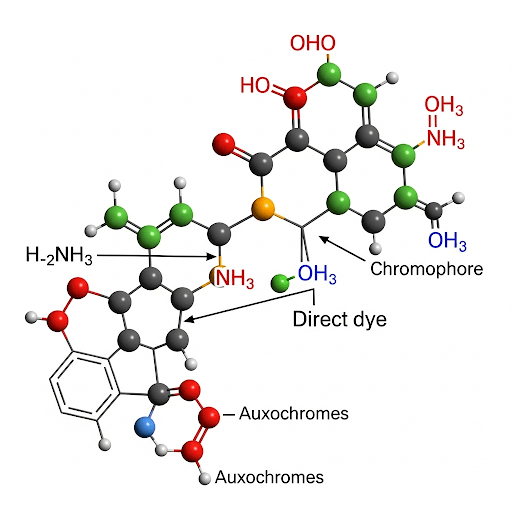
Key Structural Features:
Direct dyes are typically large, relatively linear, planar molecules. These structural characteristics are crucial for their interaction with cellulose:
- Extended Conjugated Systems: They often contain multiple azo groups (-N=N-) and other conjugated double bonds, leading to a large π-electron system responsible for color. The extended conjugation also contributes to the planarity of the molecule.
- Polar Substituents: The presence of multiple polar substituents, primarily sulfonic acid groups (-SO₃H), is essential for water solubility. These sulfonic acid groups ionize in water to form negatively charged sulfonate ions (-SO₃⁻).
- Linearity and Planarity: The elongated, flat molecular shape allows the dye molecules to align themselves along the cellulose polymer chains in the fiber. This close contact facilitates the formation of intermolecular forces.
- Auxochromic Groups: Substituents like amino (-NH₂) and hydroxyl (-OH) groups, in addition to influencing the color, also act as auxochromes, enhancing dye solubility and interaction with the fiber through hydrogen bonding.
Interaction with Cellulosic Fibers:
The dyeing mechanism of direct dyes on cellulose primarily involves:
- Substantivity: This refers to the inherent affinity of the dye for the fiber. The long, linear shape of direct dye molecules allows them to become oriented parallel to the long chains of cellulose polymers within the fiber.
- Hydrogen Bonding: The numerous hydroxyl groups in cellulose and the polar substituents (amino, hydroxyl) in the direct dye molecule can form multiple hydrogen bonds, contributing significantly to dye uptake and fixation.
- Van der Waals Forces: The close alignment of the large, planar dye molecules with the cellulose chains also allows for the cumulative effect of weak van der Waals forces to contribute to the dye-fiber interaction.
- Electrostatic Interactions: While the dye is anionic (due to sulfonic acid groups) and cellulose can develop a slight negative charge in water, the addition of electrolytes (like sodium chloride or sodium sulfate) plays a crucial role in overcoming this electrostatic repulsion. The high concentration of salt ions in the dyebath neutralizes the charges on both the fiber and the dye, allowing the dye molecules to approach the fiber surface and interact through hydrogen bonding and van der Waals forces.
Synthesis:
Direct dyes are synthesized using multiple diazotization and coupling reactions to build up the large, linear molecule with the required azo groups and substituents. The choice of aromatic amines and coupling agents determines the final color and properties of the dye.
Examples of Direct Dyes:
Many direct dyes have complex chemical structures. Some common structural motifs include:
- Dyes based on benzidine or its derivatives (though some benzidine-based dyes are now restricted due to potential health concerns).
- Dyes containing multiple azo linkages and sulfonic acid groups.
- Examples include Direct Red 81, Direct Blue 1, and Direct Yellow 12.
Fastness Properties:
While direct dyes are relatively inexpensive and easy to apply, their wash fastness and light fastness can vary depending on the specific dye structure and the depth of shade. After-treatments with cationic dye-fixing agents can improve wash fastness by forming a complex between the anionic dye and the cationic fixing agent, making the dye less soluble in water.
In Summary:
Direct dyes are a class of anionic azo dyes with specific structural features – linearity, planarity, extended conjugation, and polar substituents – that enable them to directly dye cellulosic fibers through a combination of substantivity, hydrogen bonding, van der Waals forces, and the controlled use of electrolytes.
