Lecture #17: A Deeper Dive into Dye Chemistry – Part 3: Reactive Dyes
Reactive dyes are a revolutionary class of dyes that form a covalent bond with the textile fiber. This covalent bond is the key differentiator and the reason why reactive dyes offer significantly superior wet fastness properties compared to direct dyes. They are primarily used for cellulosic fibers but also find application on protein fibers like wool and silk.
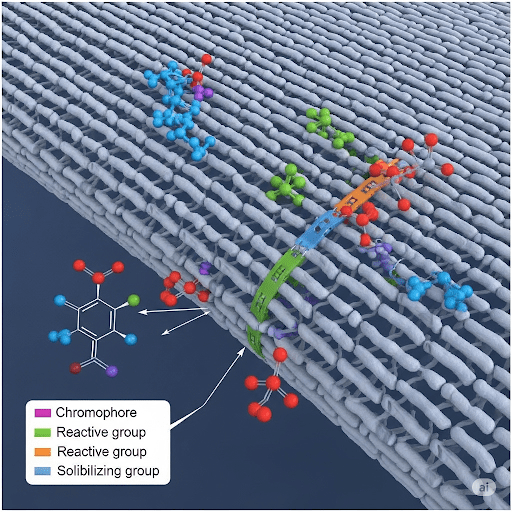
1. Introduction to Reactive Dyes
- Definition: Reactive dyes are highly colored organic substances that contain a reactive group capable of forming a covalent bond with the hydroxyl groups of cellulosic fibers (cotton, rayon) or the amino, thiol, or hydroxyl groups of protein fibers (wool, silk, nylon).
- Key Feature: The formation of a covalent bond makes the dye molecule an integral part of the fiber polymer, resulting in excellent wash fastness and good all-around fastness properties.
- Discovery: The first reactive dye, Procion M, was introduced by ICI (Imperial Chemical Industries) in 1956, marking a significant breakthrough in textile dyeing technology.
2. Chemical Structure of Reactive Dyes
The general structure of a reactive dye can be conceptualized as having three main parts:
- Chromophore: The color-imparting part of the molecule. Common chromophores include azo, anthraquinone, and phthalocyanine systems.
- Solubilizing Group: Groups like sulfonic acid (−SO3Na) and carboxyl groups (−COOH) are present to make the dye water-soluble, allowing it to penetrate the fiber from an aqueous dye bath.
- Reactive Group: This is the most crucial part, responsible for forming the covalent bond with the fiber. Reactive groups are electrophilic, meaning they are electron-deficient and readily react with nucleophilic groups (electron-rich) on the fiber (e.g., -OH on cellulose, -NH$_2$ on wool/silk).
General Structure Representation:
Dye−Bridge−Reactive Group
Where “Dye” represents the chromophore with solubilizing groups. The “Bridge” is often an amino linkage or an alkyl chain that connects the chromophore to the reactive group.
3. Common Types of Reactive Groups
Reactive dyes are primarily classified by their reactive groups, which dictate their reactivity, dyeing conditions, and fastness properties.
- a) Halotriazine Derivatives: These are the most common and commercially significant reactive groups. They contain highly activated chlorine atoms that are susceptible to nucleophilic attack.
- Monochlorotriazine (MCT) / Dichlorotriazine (DCT):
- Dichlorotriazine (Procion M dyes): These are highly reactive dyes, often requiring lower temperatures (30−60∘C) for dyeing. They have two reactive chlorine atoms.
- Example: A typical dichlorotriazine reactive group.
- Dye−NH−C6H4−NH−CN(Cl)=N(Cl)=N
- Monochlorotriazine (Procion H dyes): Less reactive than DCT, requiring higher temperatures (80−95∘C) for dyeing. They have one reactive chlorine atom.
- Example: A typical monochlorotriazine reactive group.
- Dye−NH−C6H4−NH−CN(Cl)=N(NHC6H5)=N (Note: the phenyl group is illustrative; it can be other groups)
- Dichlorotriazine (Procion M dyes): These are highly reactive dyes, often requiring lower temperatures (30−60∘C) for dyeing. They have two reactive chlorine atoms.
- Monochlorotriazine (MCT) / Dichlorotriazine (DCT):
- b) Vinyl Sulfone (VS) Derivatives: These dyes (e.g., Remazol dyes) exist in an unreactive form as β-sulfatoethylsulfone and are converted to the highly reactive vinyl sulfone form under alkaline conditions.
- Unreactive Form: Dye−SO2−CH2−CH2−OSO3Na
- Reactive Form (Vinyl Sulfone) formed via elimination: Dye−SO2−CH=CH2+Na2SO4+H2O This occurs in the presence of alkali. The double bond in the vinyl sulfone group is very reactive to nucleophilic attack.
- c) Halopyrimidine Derivatives: Less common than triazines, but also used.
- Example: Dichloropyrimidine.
- Dye−NH−C6H4−NH−CN(Cl)=C(Cl)=N
- d) Other Reactive Groups: Less frequently encountered but still relevant:
- Trichloropyrimidine
- Vinylamide
- Halogenated quinoxaline
- Phosphonic acid derivatives
4. Mechanism of Dyeing (How Reactive Dyes Work)
The dyeing process with reactive dyes involves three key stages:
Stage 1: Dye Diffusion and Adsorption (Substantivity)
- The dye molecules, being water-soluble, diffuse from the dye bath and are adsorbed onto the surface of the fiber.
- Similar to direct dyes, this initial adsorption relies on hydrogen bonding and Van der Waals forces.
- Electrolyte (Salt) Role: The addition of a neutral salt (e.g., NaCl or Na$_2$SO$_4$) is crucial at this stage.
- It reduces the negative charge on the fiber surface (zeta potential), overcoming the electrostatic repulsion between the anionic dye and the anionic fiber.
- It promotes the “salting out” effect, increasing the dye’s exhaustion onto the fiber by reducing its solubility in the aqueous phase.
Stage 2: Fixation (Covalent Bond Formation)
This is the defining stage for reactive dyes.
- Alkali Role: After sufficient dye exhaustion, an alkali (e.g., sodium carbonate – Na$_2$CO$_3$, sodium hydroxide – NaOH, or trisodium phosphate – Na$_3$PO$_4$) is added to the dye bath.
- The alkali increases the pH, making the dyeing environment strongly alkaline.
- For Cellulosic Fibers: The hydroxyl groups on the cellulose polymer, which are normally weakly acidic, are converted into highly reactive cellulosate anions (−CellO−) by the strong base. Cell−OH+NaOH→Cell−O−Na++H2O
- Nucleophilic Attack: These cellulosate anions are strong nucleophiles and attack the electrophilic reactive group on the dye molecule, leading to the formation of a stable covalent ether bond.
- Example: Reaction with Dichlorotriazine (DCT) Dye: Dye−Cl+Cell−O−→Dye−O−Cell+Cl− (Simplified, showing one reactive chlorine)
- Example: Reaction with Vinyl Sulfone (VS) Dye: First, the β-sulfatoethylsulfone is converted to the vinyl sulfone under alkaline conditions: Dye−SO2−CH2−CH2−OSO3Na+NaOH→Dye−SO2−CH=CH2+Na2SO4+H2O Then, the cellulosate anion attacks the vinyl sulfone group in a Michael addition reaction: Dye−SO2−CH=CH2+Cell−O−→Dye−SO2−CH2−CH2−O−Cell A stable ether linkage is formed.
Stage 3: Washing Off (Removal of Unfixed Dye)
- Despite efficient fixation, some dye molecules will react with water instead of the fiber (hydrolysis reaction) or simply remain unfixed in the dye bath.
- Hydrolysis Reaction: In the alkaline dye bath, water molecules (−OH) also act as nucleophiles and can compete with the fiber’s hydroxyl groups for reaction with the dye’s reactive group.
- Example: Hydrolysis of a monochlorotriazine dye: Dye−Cl+H2OOH−
Dye−OH+HCl The hydrolyzed dye is still water-soluble but no longer reactive and cannot form a covalent bond with the fiber.
- Example: Hydrolysis of a monochlorotriazine dye: Dye−Cl+H2OOH−
- Washing Process: Thorough washing with hot water, often with the addition of a detergent, is essential to remove all unfixed dye and hydrolyzed dye. This is critical for achieving good wash fastness and preventing staining of other textiles during subsequent washing.
5. Factors Influencing Reactive Dyeing
- Temperature:
- Exhaustion Phase: Generally, lower temperatures are preferred for exhaustion to allow for better leveling.
- Fixation Phase: Higher temperatures accelerate the reaction rate between the dye and fiber. Different reactive groups require specific temperature ranges (e.g., DCT at 30−60∘C, MCT/VS at 60−95∘C).
- pH / Alkali Type & Concentration:
- Crucial for generating the cellulosate anion and initiating the fixation reaction.
- The type and concentration of alkali depend on the dye’s reactivity. Stronger alkalis (NaOH) are used for less reactive dyes, while weaker alkalis (Na$_2$CO$_3$) are used for more reactive dyes.
- Excessive alkalinity can lead to increased dye hydrolysis.
- Salt Concentration:
- Optimized to achieve good exhaustion without causing aggregation or precipitation of the dye.
- Liquor Ratio: The ratio of the weight of the dye bath to the weight of the fabric. Affects dye concentration and exhaustion.
- Dye Concentration: Determines the depth of shade.
- Time: Sufficient time is required for both exhaustion and fixation.
6. Advantages and Disadvantages of Reactive Dyes
Advantages:
- Excellent Wet Fastness: Superior wash fastness, perspiration fastness, and rub fastness due to the covalent bond formation.
- Bright Shades: Produce a wide range of brilliant and vibrant colors.
- Good Lightfastness: Generally good to very good resistance to fading from light exposure.
- Versatility: Applicable to various cellulosic fibers (cotton, linen, rayon), and also wool, silk, and nylon.
- Good Reproducibility: Relatively good consistency in shade reproduction.
Disadvantages:
- Higher Cost: Generally more expensive than direct dyes.
- Complex Dyeing Process: Requires more precise control of temperature, pH, and salt concentration compared to direct dyes.
- Lower Exhaustion/Fixation Efficiency: Due to the competitive hydrolysis reaction, not all dye applied will fix to the fiber. This can lead to a significant amount of hydrolyzed dye in the effluent, posing environmental challenges.
- Requires Thorough Washing Off: The presence of unfixed and hydrolyzed dye necessitates extensive rinsing and soaping to achieve good fastness, which consumes water and energy.
- Alkali Sensitivity of Fiber: Strong alkaline conditions can sometimes damage or weaken certain fibers (e.g., some wools).
7. Classification of Reactive Dyes (Based on Reactivity)
Beyond the reactive group, reactive dyes are often classified by their overall reactivity and temperature requirements:
- Cold-Dyeing (High Reactivity) Dyes:
- Example: Procion M dyes (Dichlorotriazines).
- Require lower temperatures (30−60∘C) and milder alkalis (e.g., soda ash).
- Faster fixation, suitable for pad-batch and continuous dyeing methods.
- Warm-Dyeing (Medium Reactivity) Dyes:
- Example: Procion H dyes (Monochlorotriazines), Remazol dyes (Vinyl Sulfones).
- Require higher temperatures (60−95∘C) and stronger alkalis.
- Slower fixation, suitable for exhaust dyeing processes.
- Hot-Dyeing (Low Reactivity) Dyes:
- Less common, require very high temperatures.
8. Eco-Considerations with Reactive Dyes
While offering excellent fastness, the hydrolysis of reactive dyes can lead to high color content in wastewater. This has driven research into:
- Low-Salt Dyes: Reactive dyes designed to require less salt for exhaustion.
- Bifunctional Reactive Dyes: Dyes with two different reactive groups or two identical reactive groups. This increases the probability of one of the reactive groups forming a covalent bond with the fiber, thus improving fixation efficiency and reducing hydrolysis.
- Example: A dye containing both a monochlorotriazine and a vinyl sulfone group.
- Enzyme-assisted washing off: Using enzymes to degrade hydrolyzed dyes.
Reactive dyes remain the workhorse for high-quality, vibrant, and fast-colored cellulosic textiles, a testament to their unique chemistry and the strong covalent bond they form with the fiber.
