Textile Pretreatment: Lecture #5 – Bleaching
Welcome back to our series on Textile Pretreatment! We’ve systematically worked our way through greige fabric inspection, singeing, desizing, and scouring. Now that the fabric is clean and absorbent, it’s time to tackle its natural color, which brings us to Bleaching.
1. Introduction to Bleaching
Bleaching is a chemical process aimed at destroying or modifying the natural coloring matter present in textile fibers, rendering them white. This is a crucial step for fabrics destined for white goods, pastel shades, or bright, vibrant colors, as residual yellowish tint can dull the final shade.
Why is Bleaching Essential?
- Achieve Desired Whiteness: The primary goal is to achieve a uniformly high degree of whiteness, which is critical for white finished goods (e.g., bed sheets, medical textiles) or as a base for light and bright dye shades.
- Remove Natural Coloring Matter: Natural fibers like cotton contain natural pigments (e.g., gossypol in cotton) that give them a yellowish or brownish tint. These pigments are largely resistant to scouring and need to be chemically altered.
- Improve Color Brilliance: A perfectly white base allows dyes to achieve their true brilliance and luminosity. Any residual yellow will shift the perceived hue of a pastel or bright color.
- Ensure Uniformity: Bleaching helps to achieve uniform whiteness across the fabric, which translates to better level dyeing later.
- Final Cleaning: While not its primary purpose, bleaching also contributes to the removal of any remaining trace impurities.
2. Chemistry of Bleaching Agents (for Cellulosic Fibers)
Bleaching agents are oxidizing or reducing agents that chemically react with the chromophoric (color-imparting) groups of the natural pigments, breaking them down into colorless compounds. For cellulosic fibers like cotton, oxidizing agents are predominantly used.
a) Hydrogen Peroxide (H2O2):
- Most Common and Preferred: Hydrogen peroxide is by far the most widely used bleaching agent for cotton and other cellulosic fibers due to its effectiveness, environmental friendliness (its byproducts are water and oxygen), and the high quality of whiteness it produces.
- Mechanism: In alkaline conditions (typically pH 10-11.5), hydrogen peroxide decomposes to release nascent oxygen (O) and hydroxyl radicals (OH⋅), which are powerful oxidizing species. These species attack the conjugated double bonds of the chromophoric impurities, breaking them down into smaller, colorless, and water-soluble compounds. H2O2Alkali
H2O+O (Nascent Oxygen) H2O2Alkali
HOO−+H+ HOO−
OH⋅+O2+e− These highly reactive species then react with the natural pigments.
- Components of a Peroxide Bleaching Bath:
- Hydrogen Peroxide (H2O2): The active bleaching agent.
- Alkali (e.g., NaOH): Activates the peroxide and maintains the required pH for its decomposition and effectiveness.
- Stabilizers: Crucial to control the decomposition rate of hydrogen peroxide. Without stabilizers, peroxide decomposes too rapidly, leading to wasted chemical, uneven bleaching, and severe fiber damage (oxy-cellulose formation). Common stabilizers include sodium silicate (water glass), organic stabilizers (e.g., polyaminocarboxylic acids, phosphates). They function by complexing metal ions (which catalyze peroxide breakdown) and by buffering the pH.
- Wetting Agents/Surfactants: To ensure uniform penetration of the bleaching liquor into the fabric.
- Sequestering Agents: To chelate metal ions that can catalyze peroxide decomposition and cause fiber damage. (Often, these are part of the stabilizer package).
b) Sodium Hypochlorite (NaOCl, Chlorine Bleach):
- Older Method: Historically, hypochlorite was widely used, particularly for cotton.
- Mechanism: In aqueous solution, hypochlorite forms hypochlorous acid (HOCl), which is the active bleaching agent. It oxidizes the coloring matter.
- Disadvantages:
- Less gentle on cellulose fibers; can lead to oxy-cellulose formation and significant fiber degradation if not carefully controlled (acid tendering in subsequent steps).
- Produces undesirable byproducts, including absorbable organic halogens (AOX) in the wastewater, which are environmentally harmful.
- Lower whiteness achievable compared to peroxide.
- Usage: Less common now due to environmental concerns and the superior performance of hydrogen peroxide. If used, it requires a thorough anti-chlor (e.g., sodium bisulfite) treatment afterward to remove residual chlorine.
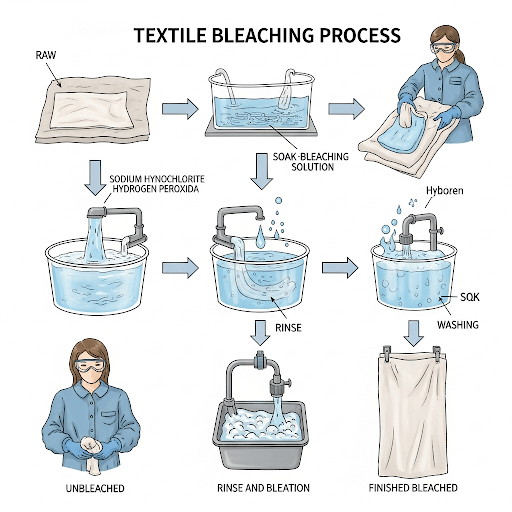
3. Bleaching Process
Bleaching can be performed in both batch and continuous modes, often integrated with scouring.
- Batch Bleaching: Done in jiggers, winches, or jets. The fabric is immersed in the bleaching liquor for several hours at elevated temperatures (e.g., 95−100∘C).
- Continuous Bleaching (Pad-Steam Method): The most common method for large volumes.
- Impregnation: Fabric is padded through a bath containing the bleaching liquor (peroxide, alkali, stabilizer, wetting agent).
- Steaming: The impregnated fabric enters a steamer where it is subjected to saturated steam (100−102∘C) for a short dwell time (minutes). The steam activates the bleaching chemicals and ensures rapid, uniform reaction.
- Washing: The fabric is thoroughly washed in multiple wash boxes to remove all residual chemicals and dissolved impurities.
- Neutralization: If an alkaline bleach, the fabric is often neutralized with a dilute acid (e.g., acetic acid) to prevent fiber damage and prepare for subsequent processes.
4. Bleaching of Other Fibers
- Polyester/Synthetic Fibers: Generally, synthetic fibers like polyester and nylon are inherently white and do not require bleaching in the traditional sense. If they are slightly off-white or need extra brightness, they might be treated with Optical Brightening Agents (OBAs), which are not bleaches but fluorescent dyes that absorb UV light and re-emit visible blue light, making the fabric appear whiter and brighter.
- Wool/Silk (Protein Fibers): These fibers are sensitive to strong oxidizing agents and alkaline conditions (which can cause yellowing and degradation).
- Reducing Bleaches: Often used for wool and silk, e.g., sodium hydrosulfite or thiourea dioxide.
- Mild Oxidizing Bleaches: Hydrogen peroxide can be used carefully under controlled, near-neutral or slightly acidic conditions with specific stabilizers. Peracetic acid is another option.
5. Testing for Bleaching Efficiency
- Whiteness Index: Measured quantitatively using a spectrophotometer (e.g., CIE Whiteness, Ganz Whiteness).
- Absorbency Test: While mostly achieved during scouring, bleaching should maintain or further enhance absorbency.
- Tensile Strength/Degree of Polymerization (DP): Important for monitoring fiber degradation. Excessive bleaching or uncontrolled peroxide decomposition can reduce the fiber’s strength.
6. Environmental and Safety Considerations
- Effluent: Bleaching effluent contains residual chemicals (peroxide, alkali, stabilizers) and oxidized organic matter. Neutralization and proper wastewater treatment are essential.
- Chemical Handling: Proper handling of strong alkalis and hydrogen peroxide is crucial for safety.
- Water and Energy Consumption: Bleaching is a water and energy-intensive process.
Bleaching is a vital step in textile pretreatment, providing the clean, uniformly white base essential for achieving high-quality colored or white textile products. The careful control of chemicals and process parameters is paramount to avoid fiber damage while achieving the desired level of whiteness.
This concludes Lecture #5 on Bleaching. Our next step in pretreatment will be Mercerization, a process specifically for cotton that enhances its strength, luster, and dye uptake.
Do you have any questions about bleaching, or shall we proceed to Mercerization?
Textile Pretreatment: Lecture #5 – Bleaching
Welcome back to our series on Textile Pretreatment! We’ve systematically worked our way through greige fabric inspection, singeing, desizing, and scouring. Now that the fabric is clean and absorbent, it’s time to tackle its natural color, which brings us to Bleaching.
1. Introduction to Bleaching
Bleaching is a chemical process aimed at destroying or modifying the natural coloring matter present in textile fibers, rendering them white. This is a crucial step for fabrics destined for white goods, pastel shades, or bright, vibrant colors, as residual yellowish tint can dull the final shade.
Why is Bleaching Essential?
- Achieve Desired Whiteness: The primary goal is to achieve a uniformly high degree of whiteness, which is critical for white finished goods (e.g., bed sheets, medical textiles) or as a base for light and bright dye shades.
- Remove Natural Coloring Matter: Natural fibers like cotton contain natural pigments (e.g., gossypol in cotton) that give them a yellowish or brownish tint. These pigments are largely resistant to scouring and need to be chemically altered.
- Improve Color Brilliance: A perfectly white base allows dyes to achieve their true brilliance and luminosity. Any residual yellow will shift the perceived hue of a pastel or bright color.
- Ensure Uniformity: Bleaching helps to achieve uniform whiteness across the fabric, which translates to better level dyeing later.
- Final Cleaning: While not its primary purpose, bleaching also contributes to the removal of any remaining trace impurities.
2. Chemistry of Bleaching Agents (for Cellulosic Fibers)
Bleaching agents are oxidizing or reducing agents that chemically react with the chromophoric (color-imparting) groups of the natural pigments, breaking them down into colorless compounds. For cellulosic fibers like cotton, oxidizing agents are predominantly used.
a) Hydrogen Peroxide (H2O2):
- Most Common and Preferred: Hydrogen peroxide is by far the most widely used bleaching agent for cotton and other cellulosic fibers due to its effectiveness, environmental friendliness (its byproducts are water and oxygen), and the high quality of whiteness it produces.
- Mechanism: In alkaline conditions (typically pH 10-11.5), hydrogen peroxide decomposes to release nascent oxygen (O) and hydroxyl radicals (OH⋅), which are powerful oxidizing species. These species attack the conjugated double bonds of the chromophoric impurities, breaking them down into smaller, colorless, and water-soluble compounds. H2O2Alkali
H2O+O (Nascent Oxygen) H2O2Alkali
HOO−+H+ HOO−
OH⋅+O2+e− These highly reactive species then react with the natural pigments.
- Components of a Peroxide Bleaching Bath:
- Hydrogen Peroxide (H2O2): The active bleaching agent.
- Alkali (e.g., NaOH): Activates the peroxide and maintains the required pH for its decomposition and effectiveness.
- Stabilizers: Crucial to control the decomposition rate of hydrogen peroxide. Without stabilizers, peroxide decomposes too rapidly, leading to wasted chemical, uneven bleaching, and severe fiber damage (oxy-cellulose formation). Common stabilizers include sodium silicate (water glass), organic stabilizers (e.g., polyaminocarboxylic acids, phosphates). They function by complexing metal ions (which catalyze peroxide breakdown) and by buffering the pH.
- Wetting Agents/Surfactants: To ensure uniform penetration of the bleaching liquor into the fabric.
- Sequestering Agents: To chelate metal ions that can catalyze peroxide decomposition and cause fiber damage. (Often, these are part of the stabilizer package).
b) Sodium Hypochlorite (NaOCl, Chlorine Bleach):
- Older Method: Historically, hypochlorite was widely used, particularly for cotton.
- Mechanism: In aqueous solution, hypochlorite forms hypochlorous acid (HOCl), which is the active bleaching agent. It oxidizes the coloring matter.
- Disadvantages:
- Less gentle on cellulose fibers; can lead to oxy-cellulose formation and significant fiber degradation if not carefully controlled (acid tendering in subsequent steps).
- Produces undesirable byproducts, including absorbable organic halogens (AOX) in the wastewater, which are environmentally harmful.
- Lower whiteness achievable compared to peroxide.
- Usage: Less common now due to environmental concerns and the superior performance of hydrogen peroxide. If used, it requires a thorough anti-chlor (e.g., sodium bisulfite) treatment afterward to remove residual chlorine.
3. Bleaching Process
Bleaching can be performed in both batch and continuous modes, often integrated with scouring.
- Batch Bleaching: Done in jiggers, winches, or jets. The fabric is immersed in the bleaching liquor for several hours at elevated temperatures (e.g., 95−100∘C).
- Continuous Bleaching (Pad-Steam Method): The most common method for large volumes.
- Impregnation: Fabric is padded through a bath containing the bleaching liquor (peroxide, alkali, stabilizer, wetting agent).
- Steaming: The impregnated fabric enters a steamer where it is subjected to saturated steam (100−102∘C) for a short dwell time (minutes). The steam activates the bleaching chemicals and ensures rapid, uniform reaction.
- Washing: The fabric is thoroughly washed in multiple wash boxes to remove all residual chemicals and dissolved impurities.
- Neutralization: If an alkaline bleach, the fabric is often neutralized with a dilute acid (e.g., acetic acid) to prevent fiber damage and prepare for subsequent processes.
4. Bleaching of Other Fibers
- Polyester/Synthetic Fibers: Generally, synthetic fibers like polyester and nylon are inherently white and do not require bleaching in the traditional sense. If they are slightly off-white or need extra brightness, they might be treated with Optical Brightening Agents (OBAs), which are not bleaches but fluorescent dyes that absorb UV light and re-emit visible blue light, making the fabric appear whiter and brighter.
- Wool/Silk (Protein Fibers): These fibers are sensitive to strong oxidizing agents and alkaline conditions (which can cause yellowing and degradation).
- Reducing Bleaches: Often used for wool and silk, e.g., sodium hydrosulfite or thiourea dioxide.
- Mild Oxidizing Bleaches: Hydrogen peroxide can be used carefully under controlled, near-neutral or slightly acidic conditions with specific stabilizers. Peracetic acid is another option.
5. Testing for Bleaching Efficiency
- Whiteness Index: Measured quantitatively using a spectrophotometer (e.g., CIE Whiteness, Ganz Whiteness).
- Absorbency Test: While mostly achieved during scouring, bleaching should maintain or further enhance absorbency.
- Tensile Strength/Degree of Polymerization (DP): Important for monitoring fiber degradation. Excessive bleaching or uncontrolled peroxide decomposition can reduce the fiber’s strength.
6. Environmental and Safety Considerations
- Effluent: Bleaching effluent contains residual chemicals (peroxide, alkali, stabilizers) and oxidized organic matter. Neutralization and proper wastewater treatment are essential.
- Chemical Handling: Proper handling of strong alkalis and hydrogen peroxide is crucial for safety.
- Water and Energy Consumption: Bleaching is a water and energy-intensive process.
Bleaching is a vital step in textile pretreatment, providing the clean, uniformly white base essential for achieving high-quality colored or white textile products. The careful control of chemicals and process parameters is paramount to avoid fiber damage while achieving the desired level of whiteness.
