extile Dyeing and Dyes: Lecture #3 – The Heart of Dyeing: Understanding Dye-Fiber Interactions
Welcome back to our series on Textile Dyeing and Dyes! In our previous lecture, we explored the classification of dyes based on their chemical structure and application methods. Building upon that foundation, today we will delve into the core of the dyeing process: the interactions between dye molecules and textile fibers. Understanding these interactions at a molecular level is crucial for comprehending why specific dyes exhibit affinity for certain fibers and the factors that contribute to color fastness.
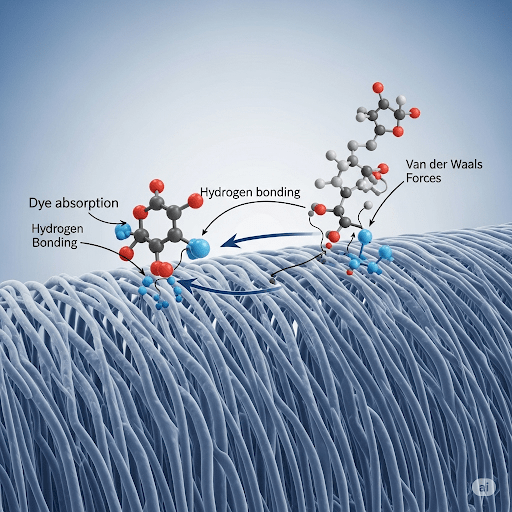
The successful dyeing of a textile fiber relies on the dye molecules being able to:
- Be attracted to the fiber surface (Adsorption).
- Penetrate into the fiber structure (Diffusion).
- Be held within the fiber with sufficient tenacity (Fixation).
The nature of these steps is largely governed by the chemical and physical properties of both the dye and the fiber. Let’s explore the primary types of interactions involved:
1. Ionic Bonds (Electrostatic Attractions):
- Mechanism: These bonds occur between oppositely charged ions. Fibers like wool, silk, and nylon contain amino (-NH₂) and carboxyl (-COOH) groups that can ionize depending on the pH of the dyebath, acquiring positive or negative charges. Acid dyes are typically anionic (negatively charged), while basic dyes are cationic (positively charged). The electrostatic attraction between these oppositely charged dye and fiber sites leads to dye uptake.
- Fibers Involved: Primarily protein fibers (wool, silk) and polyamide fibers (nylon).
- Dye Classes: Acid dyes (anionic) and Basic dyes (cationic).
- Detail: In an acidic dyebath, the amino groups in wool, silk, and nylon become protonated (-NH₃⁺), developing a positive charge. This positive charge attracts the negatively charged sulfonate (-SO₃⁻) or carboxylate (-COO⁻) groups present in acid dye molecules, forming ionic bonds. Conversely, basic dyes with positively charged amino groups are attracted to the negatively charged sites that can be created on certain fibers under specific conditions.
- Contribution to Fastness: Ionic bonds provide a reasonably strong attraction, contributing to moderate to good wash fastness, depending on the strength of the charges and the size of the dye molecule.
2. Hydrogen Bonds:
- Mechanism: Hydrogen bonds are weaker intermolecular forces that occur between a hydrogen atom bonded to a highly electronegative atom (such as oxygen or nitrogen) and another electronegative atom with a lone pair of electrons. Many textile fibers, particularly natural and regenerated cellulosic fibers (cotton, viscose, linen) and protein fibers (wool, silk), contain hydroxyl (-OH) and amino (-NH) groups that can participate in hydrogen bond formation. Direct dyes, for example, often have structures that allow them to align with the cellulose polymer chains and form multiple hydrogen bonds.
- Fibers Involved: Cellulosic fibers (cotton, viscose, linen), protein fibers (wool, silk), and some synthetic fibers (e.g., nylon with its amide groups).
- Dye Classes: Direct dyes, some reactive dyes, and some acid dyes can form hydrogen bonds with fibers.
- Detail: Direct dyes, often long, planar molecules with multiple azo groups and auxochromes like amino and hydroxyl groups, can align themselves along the cellulose chains in cotton and viscose. The hydroxyl groups of cellulose and the azo and amino groups of the dye molecules form numerous hydrogen bonds, leading to dye uptake.
- Contribution to Fastness: While individually weak, the cumulative effect of a large number of hydrogen bonds can contribute significantly to dye fixation and provide moderate wash fastness.
3. Van der Waals Forces (London Dispersion Forces):
- Mechanism: These are weak, short-range attractive forces that arise from temporary fluctuations in electron distribution around molecules, creating temporary dipoles that induce dipoles in neighboring molecules. These forces are significant when dye molecules can come into close contact with the fiber surface, which is often facilitated by the size and shape of the dye molecule and the packing of the fiber polymers. Disperse dyes, used for hydrophobic synthetic fibers, rely heavily on van der Waals forces.
- Fibers Involved: Hydrophobic synthetic fibers like polyester, acetate, and nylon.
- Dye Classes: Primarily Disperse dyes.
- Detail: Disperse dyes are small, non-ionic, and relatively water-insoluble molecules. They are applied as a fine dispersion and, at high temperatures, can penetrate the hydrophobic fibers. Once inside, the dye molecules are held within the fiber matrix by van der Waals forces arising from close interactions with the polymer chains. The solubility of the dye in the fiber polymer also plays a crucial role.
- Contribution to Fastness: The effectiveness of van der Waals forces depends on the closeness of contact and the number of interacting sites. Well-dispersed dyes within the fiber structure can exhibit good wash fastness.
4. Covalent Bonds:
- Mechanism: These are strong chemical bonds formed by the sharing of electron pairs between atoms. Reactive dyes are unique in that they form covalent bonds with the fiber molecules (typically hydroxyl groups in cellulose, amino and hydroxyl groups in wool and silk). This chemical linkage creates a very strong and permanent dye-fiber bond.
- Fibers Involved: Primarily cellulosic fibers (cotton, viscose), but also protein fibers (wool, silk).
- Dye Classes: Reactive dyes.
- Detail: Reactive dyes contain a reactive group (e.g., triazine, vinyl sulfone) that can undergo a chemical reaction with functional groups present in the fiber under specific pH and temperature conditions. For example, a reactive dye with a triazine ring can react with the hydroxyl groups of cellulose in an alkaline dyebath, forming a stable ether or ester linkage.
- Contribution to Fastness: Covalent bonds are the strongest type of interaction and result in excellent wash fastness. The dye becomes an integral part of the fiber molecule.
5. Mechanical Entrapment:
- Mechanism: In some cases, dye molecules or pigments can be physically trapped within the fiber structure. Vat dyes, once reduced to their soluble leuco form and absorbed by the fiber, are then oxidized back to their insoluble form within the fiber. The large, insoluble dye molecules become physically entrapped within the fiber matrix. Pigments, being insoluble colorants, are also mechanically held onto the fiber surface using binders.
- Fibers Involved: Primarily cellulosic fibers for vat dyes; applicable to most fibers for pigments.
- Dye Classes: Vat dyes (fixation), Pigments (application).
- Detail: For vat dyeing, the soluble leuco form diffuses into the cotton fiber. Subsequent oxidation converts it back to the insoluble colored form, which is now too large to diffuse out of the fiber. Pigments, on the other hand, are not dyes in the true sense as they don’t form chemical bonds or dissolve within the fiber. They are adhered to the surface by a binding agent that forms a film around the pigment particles and attaches them to the fabric.
- Contribution to Fastness: Vat dyes exhibit excellent wash fastness due to mechanical entrapment. Pigment fastness depends heavily on the effectiveness and durability of the binder.
Conclusion:
The interaction between dye molecules and textile fibers is a complex process governed by a variety of chemical and physical forces. The type of fiber, the chemical structure of the dye, and the conditions of the dyebath all play crucial roles in determining the extent of dye uptake and the resulting color fastness. Understanding these fundamental interactions is essential for selecting the appropriate dye class for a given fiber and for optimizing the dyeing process to achieve the desired color and durability.
In our next lecture, we will begin to explore the specific dyeing processes for different fiber types, starting with the most widely used natural fiber: cotton.
