The Chemistry of Wool: Lecture #29 (in a Series on Textile Fibers)
- Introduction: Wool in the Realm of Textile Fibers
Wool stands as a time-honored and commercially significant natural protein fiber, its utility woven into the fabric of human history for millennia. In the vast and varied world of textile fibers, wool occupies a prominent position alongside its natural counterparts, such as plant-derived cellulose fibers (cotton, linen) and other animal fibers (silk, cashmere), as well as the synthetic fibers engineered in laboratories (polyester, nylon).
What distinguishes wool and endows it with enduring value are its remarkable inherent properties. These include its exceptional warmth, a testament to its insulating capabilities, its natural elasticity that allows garments to retain their shape, its impressive ability to absorb moisture while still feeling dry, and its inherent flame resistance, offering a degree of safety unmatched by many other textiles. This lecture, the twenty-eighth in our series on textile fibers, will delve into the intricate and fascinating chemistry of wool fibers, exploring the fundamental principles that underpin these valuable characteristics.
- The Fundamental Chemistry of Wool: Building Blocks and Composition
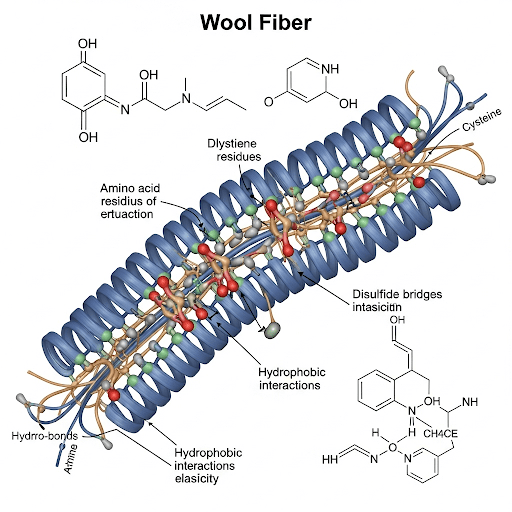
At its core, wool is predominantly composed of proteins, belonging to a specialized group known as keratins. These keratins constitute an overwhelming majority of the fiber’s weight, typically ranging from 95 to 98 percent. Like all proteins, keratins are long-chain polymers constructed from smaller units called amino acids, which are linked together in a specific sequence through peptide bonds. Wool’s keratin structure incorporates 18 of the 22 amino acids that commonly occur in nature. Among these, certain amino acids play particularly significant roles in defining wool’s unique properties. Cysteine (C3H7NO2S) , a sulfur-containing amino acid, is crucial as it enables the formation of disulfide bonds, which act as strong crosslinks within and between the protein chains. The content of cysteine in wool typically ranges from 5 to 10 mol percent. Other important amino acids present in significant amounts include glycine (C2H5NO2) , alanine (C3H7NO2) , serine (C3H7NO3) , and glutamic acid (C5H9NO4).
Beyond its proteinaceous foundation, wool also contains a small percentage of other chemical components. Lipids, including lanolin (C34H68O2) , are present at around 2 percent and contribute to wool’s inherent water-repellent characteristics. Mineral salts constitute approximately 1 percent of the fiber. Additionally, wool is hygroscopic, meaning it readily absorbs moisture from the surrounding environment, with a typical moisture regain of about 12 to 16 percent under standard atmospheric conditions.
To better understand wool’s chemistry, it is instructive to compare it with another important natural protein fiber: silk. Silk, primarily composed of the protein fibroin, shares the fundamental building blocks of amino acids linked by peptide bonds. However, the amino acid composition of silk differs significantly from that of wool. Silk fibroin is predominantly made up of glycine, alanine, and serine, with a notable absence of cysteine and thus disulfide bonds. This key chemical difference has profound implications for the structural organization and resulting properties of these two fibers.
| Amino Acid | Approximate Percentage in Wool Keratin | Approximate Percentage in Silk Fibroin |
|---|---|---|
| Glycine | 5-10 | 45 |
| Alanine | 2-5 | 29 |
| Serine | 8-12 | 12 |
| Cysteine | 5-10 | 0 |
| Glutamic Acid | 10-15 | 1 |
| Other Amino Acids | Remainder | Remainder |
Export to Sheets
This comparison highlights the unique presence of cysteine in wool and the significantly different proportions of other key amino acids in silk, providing an initial glimpse into the chemical basis of their distinct characteristics.
- A Fiber’s Architecture: The Hierarchical Structure of Wool
The remarkable properties of wool are not solely determined by its chemical composition but are also intricately linked to its complex and well-organized hierarchical structure. This structure can be viewed at multiple levels, starting from the outermost layer and progressing inwards.
The cuticle forms the fiber’s exterior, acting as a protective sheath. It is characterized by overlapping scales, composed of individual cuticle cells, resembling tiles on a roof. These scales possess a waxy outer coating known as the epicuticle, which contains 18-methyleicosanoic acid (C21H42O2). This lipid layer is responsible for wool’s ability to repel water, contributing to its shower-proof nature. The exposed edges of these scales are also the primary cause of wool’s unique felting property. The jagged surface allows the fibers to interlock when subjected to mechanical action in the presence of moisture and heat.
Beneath the cuticle lies the cortex, which constitutes the bulk of the wool fiber, approximately 90 percent of its mass. The cortex is composed of elongated, spindle-shaped cells known as cortical cells. These cells are further organized into fibrils, which are themselves composed of macrofibrils and even finer microfibrils. A key feature of the cortex in fine wools like Merino is the presence of two main types of cortical cells: ortho-cortex and para-cortex cells. These cell types exhibit slight differences in their chemical composition, which leads to variations in their behavior, particularly in how they absorb dyes and how they respond to moisture. This bilateral arrangement of ortho- and para-cortex cells is directly responsible for the natural crimp, or waviness, of wool fibers.
In some coarser wool fibers, a central core known as the medulla can be found. The medulla is a hollow network of air-filled cells, which contributes to increased light reflection, potentially affecting the fiber’s luster and color, as well as enhancing its insulation properties. However, the medulla is often absent or very small in finer grades of wool, such as Merino.
The cell membrane complex (CMC) is another crucial structural component. This continuous region, composed of proteins and waxy lipids, surrounds the cortical cells, holding them together and also forming the interface between the cortex and the cuticle. The CMC acts as a pathway through which dyes and other chemical treatments can penetrate into the wool fiber. The intermolecular bonds within the CMC are relatively weak, making it more susceptible to breakdown upon exposure to strong chemicals and prolonged abrasion.
- The Molecular Structure of Wool Keratin: Unveiling the Protein’s Form
The hierarchical structure observed at the microscopic level is a direct consequence of the molecular arrangement of the wool proteins, primarily alpha-keratins (α-keratins). The dominant secondary structure within these proteins is the alpha-helix, a coiled, spring-like conformation. This helical structure is stabilized by hydrogen bonds formed within the same polypeptide chain. Additionally, some beta-sheet structures are present, particularly in the matrix proteins that surround the more ordered helical regions.
These individual alpha-helices do not exist in isolation but are further organized into higher-order structures. Several alpha-helices intertwine to form coiled-coil structures, which then assemble into protofilaments, followed by microfibrils, and finally macrofibrils, the larger filaments visible within the cortical cells. This hierarchical arrangement contributes to the overall strength and flexibility of the wool fiber.
Crucially, the wool keratin structure is reinforced by a network of intermolecular crosslinks. The most significant of these are disulfide bonds, which form between the sulfur atoms of cysteine residues located on adjacent polypeptide chains. These covalent bonds provide considerable strength and stability to the keratin network, contributing to wool’s insolubility and its resistance to both chemical and physical stresses. In addition to disulfide bonds, salt linkages (ionic bonds between acidic and basic amino acid side chains) and hydrophobic interactions between nonpolar side chains also play a role in stabilizing the wool keratin structure.
- Structure Dictates Function: The Chemistry Behind Wool’s Physical Attributes
The intricate chemical and structural organization of wool directly gives rise to its distinctive physical properties.
The elasticity and resilience of wool are primarily attributed to the alpha-helical conformation of its keratin proteins. These spring-like structures can be stretched and will readily return to their original length once the applied force is removed. The disulfide bonds, acting as crosslinks within the protein network, play a critical role in maintaining this structure and allowing the fiber to regain its shape after deformation. The crimp inherent in wool fibers also contributes to their overall resilience, allowing them to recover from compression and bending.
Wool’s remarkable hygroscopicity, its ability to absorb moisture, arises from the presence of polar peptide groups and salt linkages within the keratin structure. These groups readily attract and bind water molecules. Wool can absorb up to 30 percent of its weight in moisture without feeling damp because the water is held within the fiber’s structure. The more amorphous regions within the wool’s polymer system also provide spaces for water molecules to penetrate and interact.
The excellent thermal insulation provided by wool is largely due to the crimp of the fibers. 1 This natural waviness creates numerous air pockets within the fabric structure, and air is a poor conductor of heat. These trapped air spaces effectively insulate the wearer against both cold and heat [ 2 , S
